- Additives
- Instruments
Generally, as potentially foam-stabilizing substances cannot be avoided in coating formulations, defoamers are used to prevent foaming despite the presence of these substances, or to destroy any foam produced as quickly as possible.
Defoamers are liquids with low surface tension that need to essentially fulfill three conditions:
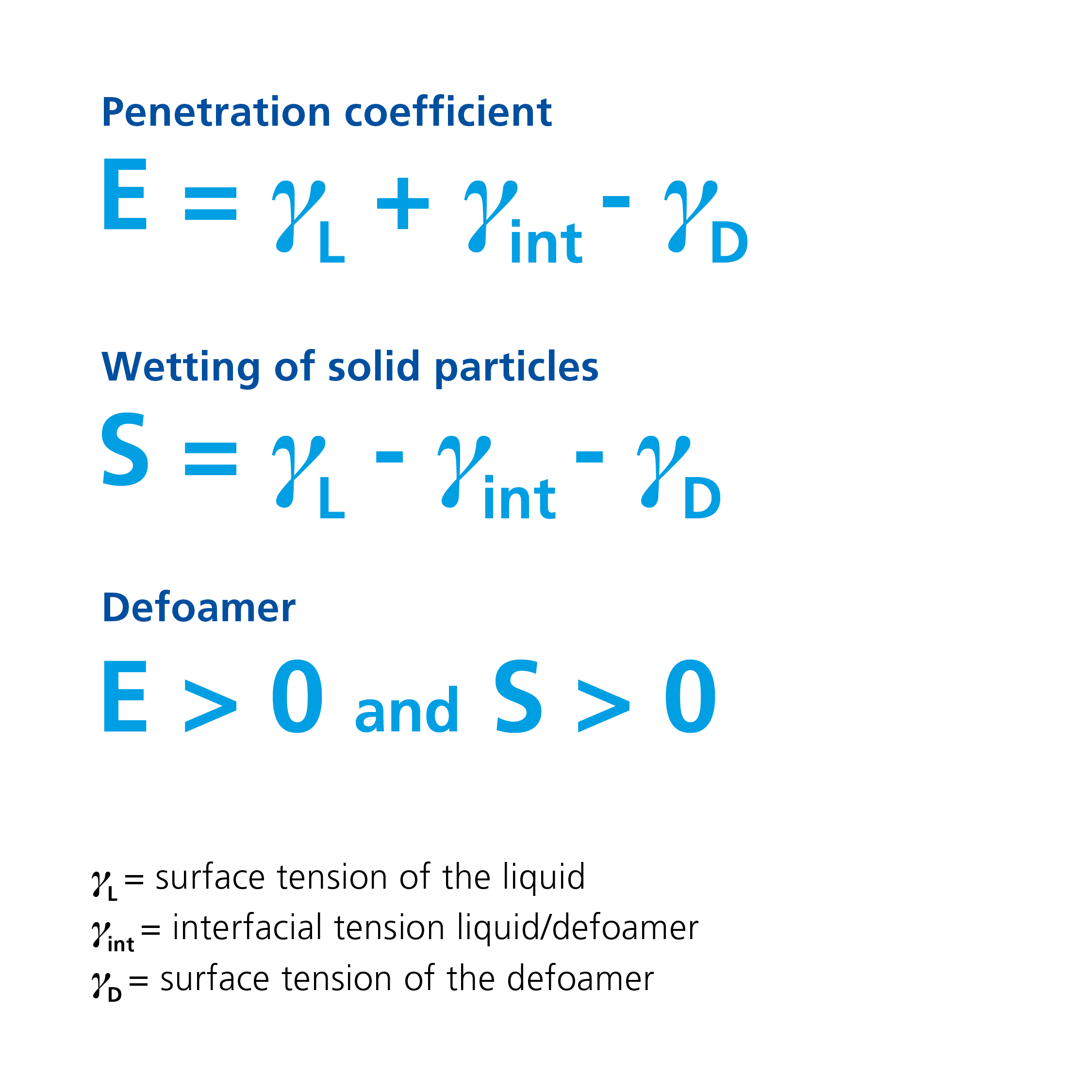
If the penetration coefficient is positive, the defoamer can penetrate the foam lamella. If the condition of a positive spreading coefficient is also met, the defoamer is able to spread at the interface after penetrating the lamella. By spreading at the interface of the foam lamella, the foam-stabilizing surfactants are displaced, and the elastic lamella that is stable to interference is replaced by a film with lower cohesive forces.
In addition to particles from hydrophobic silicas, BYK has developed two particle technologies that are based on polyureas and polyamides.
Polyurea particles are characterized by their better defoaming effect and by two further benefits:
1. Polyurea is synthesized from liquid reactants in situ in the carrier oil, which achieves substantially smaller particles and a better resistance to settling. The defoamer itself has better storage stability.
2. The adsorption capacity for surfactants is greater as a result of the larger specific surface, thereby ensuring optimum defoaming, even if the coating is stored for a long period.
Technologies based on polyamide particles are also beneficial as they can be used for applications with food contact. In certain applications they also achieve greater efficiency.
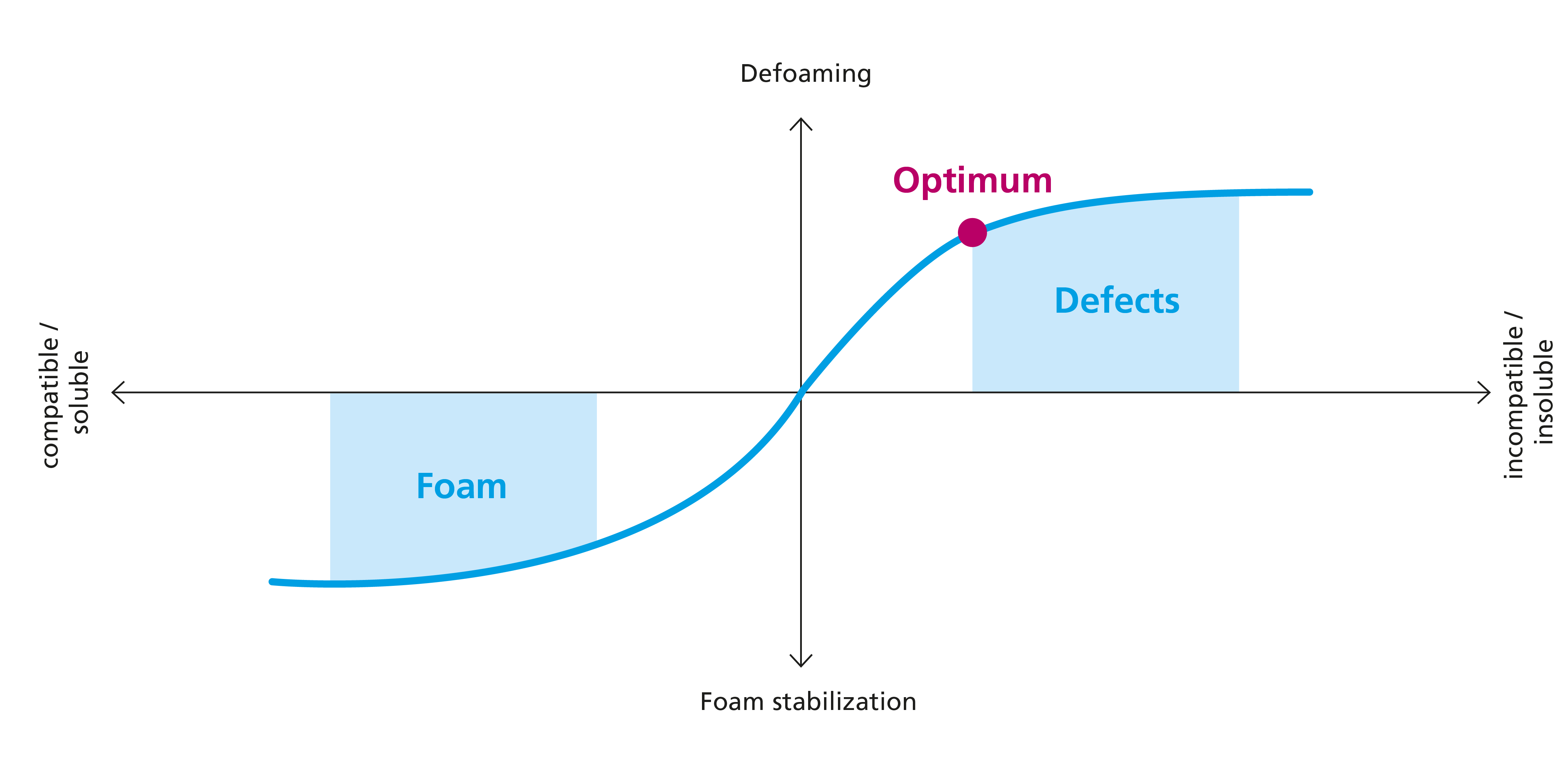
Another key feature of all defoamers is their targeted and controlled incompatibility with the medium that is to be defoamed. A defoamer that is too compatible does not migrate into the foam lamella specifically, it is present in the entire coating film; the defoaming effect is then either low or non-existent. Too much incompatibility causes problematic coating defects such as turbidity or cratering. Choosing the correct defoamer is therefore a kind of “balancing act” between compatibility and incompatibility. This situation is shown graphically in the following figure: too great compatibility causes a foam-stabilizing instead of a defoaming effect. The optimum level is found when good defoaming is achieved without defects (turbidity, cratering).
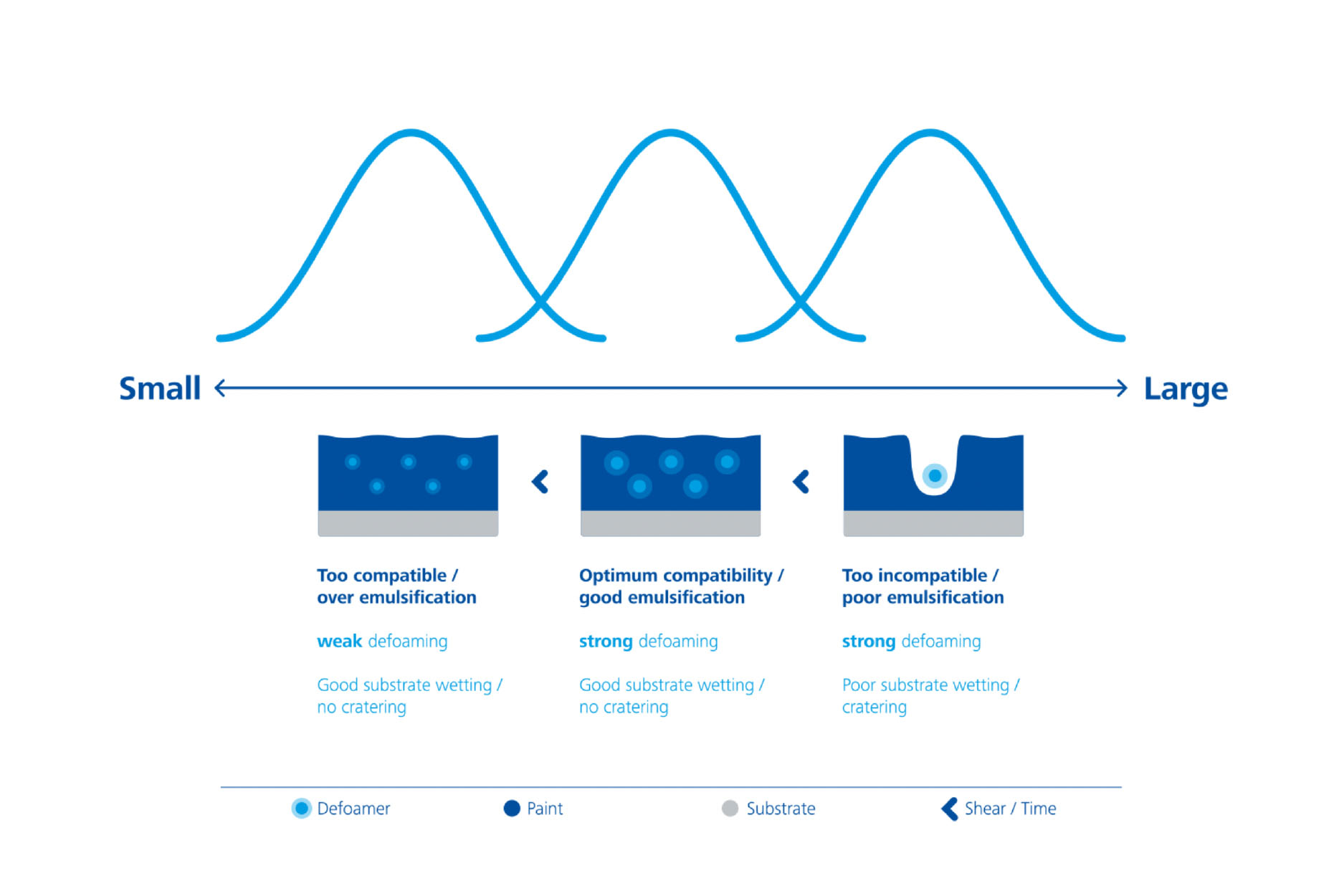
Selecting a defoamer therefore depends on the time of addition, the duration and incorporation method (shear forces), and the dosage in the coating formulation. All of these parameters have an impact on the distribution and therefore on the droplet size of the defoamer in the coating formulation. They therefore influence its effectiveness. If the dispersing process is inefficient and causes the formation of droplets that are too large, the coating suffers from surface defects. If dispersion is too great, the defoamer droplets are too small and their behavior in the coating formulation is too “compatible”, decreasing the effectiveness.
For defoamers with especially high activity, which are present as concentrate, the defoamer droplets need to be created in situ. This can be caused, for instance, by the defoamer in the coating formulation being finely distributed in the millbase at high shear force. For defoamer emulsions, in which the emulsified droplets are already finely distributed in the water, the defoamer can be added to the coating formation at the low shear forces that are usually used during the letdown.
As a result of the multitude of different coating systems, there is no “one” defoamer that is optimally suited to all formulations. To ensure that a suitable product can be provided for any purpose, a range of defoamers is required. The defoaming effect can be finely adjusted by varying the dosage: In general, better defoaming is achieved the more defoamer is used. However, this may also increase the defects or rather these may become more visible. Reducing the dosage prevents film defects, though the defoaming effect may, in some circumstances, not be sufficient.
The term “defoaming” is frequently used to describe the removal of gas bubbles from the coating. However, in certain cases the terms “defoaming” and “air release” should be differentiated. First, the gas bubbles need to reach the surface. Removing the foam bubbles that are at the surface is called defoaming. Defoamers are only effective at the surface where they eliminate the air bubbles located there. In comparison, air release agents take effect throughout the coating film.
Foam at the surface. Defoamers destabilize the foam bubbles.
Air inclusions in the coating film. Air release agents accelerate the migration of bubbles to the surface.
Small micro bubbles (microfoam) can be entrapped in the coating film. This happens particularly when the film thickness and the viscosity of the formulation is high, hindering the rising of the small bubbles to the surface (see Stokes´s law here above). The internal pressure in small bubbles is higher compared to the external pressure and this phenomenon will cause the air to diffuse out of the bubbles into the coating. During the drying process the small micro bubbles get smaller and literally shrink and disappear. This dissolution process can be followed by a camera linked to a microscope.
This diffusion process of the air ends, however, if the bubble is stabilized as a result of surfactants being adsorbed by the bubble walls. As a result of their surface activity, the mechanism of action of air release agents therefore consists of an orientation at the interface between liquid and air and in the removal of stabilizing surfactants at the interface, enabling dissolution to take place. As it is not always possible to differentiate between these two effects in practice, it can be difficult to establish to what extent an additive is effective as a defoamer or an air release agent.
For this reason, we will use the term “defoamer” in the following even though in specific cases “air release agent” would actually be the correct terminology. In the following, we will primarily illustrate the chemistry of BYK defoamers in aqueous and solvent-borne systems. They can be sub-divided into the following groups:
If you delete your search history, all your previous searches will be deleted permanently.