- Additives
- Instruments
Wetting and dispersing additives have been available for a long time now, frequently fatty acid-based with an adhesive group per molecule (anionic, cationic, non-ionogenic) and which can be classified as low-molecular weight polymers. They have a deflocculating effect and have been successfully used to stabilize inorganic pigments and are still being used today. For example, the long-established ANTI‑TERRA‑U is an additive from this group that is still widely in use. Newer developments include products like DISPERBYK‑107 or DISPERBYK‑108 which fulfill modern requirements such as containing no aromatic solvents or no solvents at all. Products such as DISPERBYK‑111 or DISPERBYK‑180 are used to stabilize titanium dioxide and inorganic pigments in the automotive sector and in industrial coating systems.
However, when it is necessary to stabilize organic pigments (or also fine-particle carbon black pigments) against flocculation, these additives show considerable weaknesses. A durable and permanent adsorption onto the pigment surface is of utmost importance in order for additives to be effective, as this is the only way that a stable protective envelope can be formed. Inorganic pigments are ionically constructed and display relatively high surface polarities, thus making adsorption of the additives relatively easy. Organic pigments have a completely different structure. Here, the pigment crystals are made up of individual molecules which are also predominantly non-polar and held together by means of intermolecular forces. As a result, organic pigments have very non-polar surfaces and therefore make proper adsorption of conventional additives rather difficult. On account of the negligible interactive forces between the adhesive groups and the pigment surface, the dispersing additives are able to fall away from this very easily and there is no stable protective envelope around the pigment particles. In practice, this means that in many cases organic pigments are insufficiently deflocculated and stabilized using low-molecular weight wetting and dispersing additives. Moreover, this is compounded by the fact that the fine-particle organic pigments have more of a tendency to flocculate than the coarse-particle inorganic ones. As the entire pigment surface must be covered with additive molecules and, on account of their smaller particle size, the organic pigments have a greater specific surface, considerably higher additive doses are required. High additive quantities can have a negative effect on the coating film properties (e.g. hardness, water-resistance).
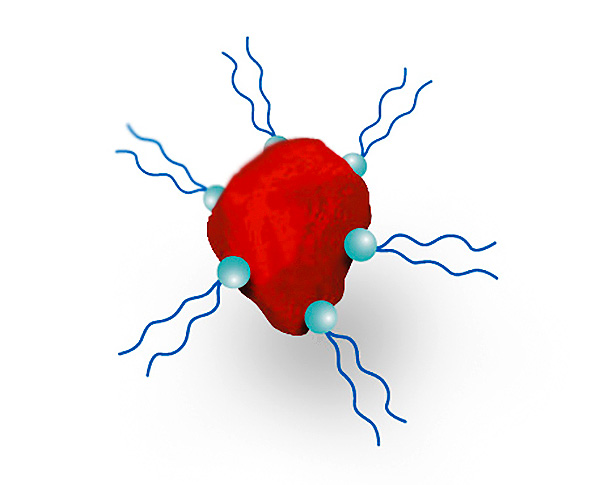
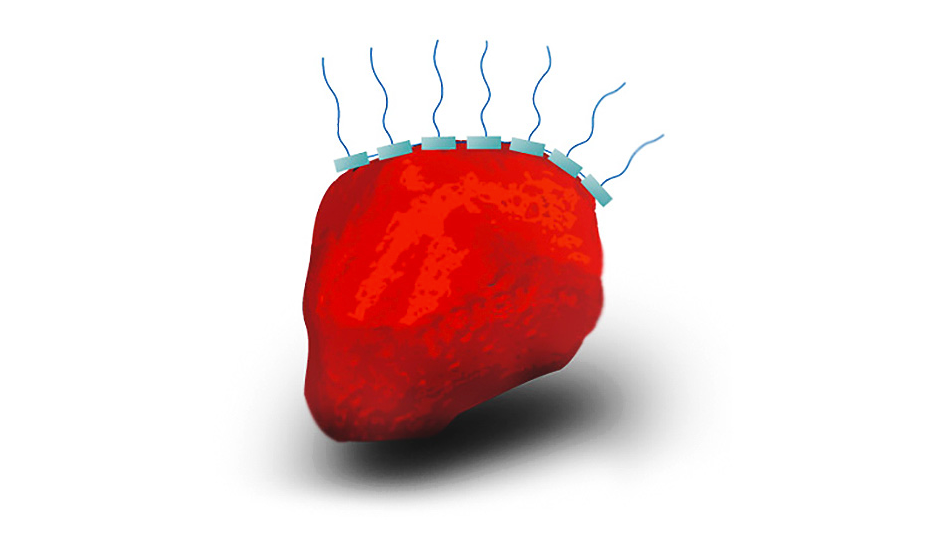
Polymer wetting and dispersing additives: a multitude of anchor groups ensure good adsorption even on less polar pigment surfaces, e.g. in the case of organic pigments. The highly solvated polymer segments, which are also protruding from the pigment surface, cause a steric stabilization of the pigments.
For the most varied of reasons, in the past increasingly more organic pigments have been used (e.g. for heavy metal-free, more brilliant shades) and this trend has led to the development of a new group of additives: polymeric wetting and dispersing additives. They differentiate themselves from conventional low-molecular weight products primarily by two structural features: on the one hand, they have a considerably greater molecular mass and thereby a character similar to that of a binder. However, that is just a side effect; what is more significant is that these additives contain a very large number of adhesive groups. The molecule has to be enlarged in order to accommodate all these adhesive groups. Even if the adsorption of an adhesive group on the pigment surface is only weak, the large number of contact points between the additive and the pigment achieves a stable, durable adsorption even on organic pigments. These additives develop their stabilizing effect – the same way as conventional products – by steric hindrance as a result of the polymer segments which are protruding into the binder solution.
Optimal stabilization is possible only when such polymer chains are properly unfurled and therefore quite compatible with the surrounding polymer solution. If this compatibility is restricted, the polymer chains collapse. Consequently, all chances for steric hindrance and the resultant stabilization are lost. The compatibility of highly polymeric additives with various coating binders is considerably more restricted than that of a low-molecular weight variety. Accordingly, an entire family of chemically related additives (classified according to molecular weight, polarity and compatibility) is available.
Segments with adhesive groups and binder-compatible segments in the form of polymer loops and chains can be combined with each other in a variety of ways. Statistical blocking and plugging copolymers have already proven successful. A number of factors are important for a good steric stabilization of the deflocculated pigments. The molecular mass and the molecular mass distribution play a key role alongside the polymer architecture.
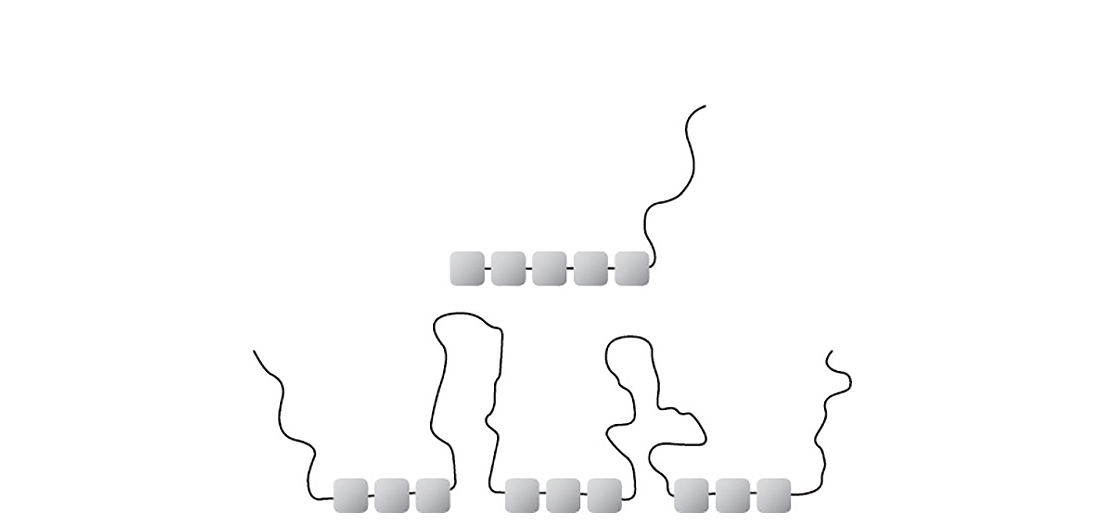
Different combinations of segments with adhesive groups and binder-compatible side chains are possible with polymeric wetting and dispersing additives.
The high-molecular weight wetting and dispersing additives have indeed been specially developed for organic pigments, but are actually just as suitable for inorganic pigments and particularly also for stabilizing pigment blends.
Major additives in this group are DISPERBYK‑161 for high-quality industrial coatings such as automotive coatings and DISPERBYK‑2163 or DISPERBYK‑2164 (no aromatic substances) for a wide range of industrial applications. These additives contain cationic anchor groups which, in isolated cases, may interact with acidic components in the coating formulation (e.g. acid catalysts in coil coatings). In such cases, additives from our DISPERBYK‑170 or DISPERBYK‑174 families are recommended. They make use of a different adhesive group chemistry and thus avoid such problems. In addition, there are modern branched structures with sterically hindered cationic adhesive groups (DISPERBYK‑2155) or complex core shell polymers where the pigment-affinic block is encapsulated (DISPERBYK‑2152). This reduces the reactivity of the additives in view of the coating systems to a minimum with the constant effectiveness in the stabilization of inorganic and organic pigments as well as carbon blacks.
In aqueous systems which are based on emulsion binders and primarily for emulsion paints and plasters which are used in the area of architectural coatings, the pigments are predominantly stabilized by electrostatic repulsion. Ammonium salts of polycarboxylic acids (such as BYK‑154) are frequently used.
In principle, aqueous systems that are based on water-soluble binder solutions or combinations of such binder solutions with binder solution emulsions (hybrid systems) can also use electrostatic repulsion for pigment stabilization. However, in practice it is found that steric stabilization with polymeric wetting and dispersing additives is frequently preferred, especially in high-quality industrial coatings. The mechanism works the same way as in solvent-borne coatings, the only requirement being that the polymeric additives must be polar enough to ensure compatibility with the aqueous surroundings. It is not necessarily desirable for such additives to be water-soluble since too high a polarity could adversely affect the durability of the coating film (e.g. water-resistance). Typical products representing this additive group are DISPERBYK‑184, DISPERBYK‑190 and DISPERBYK‑194 N.
If you delete your search history, all your previous searches will be deleted permanently.